21.06.2012
Unstable tin-100 for the first time investigated in detail
Shortly after the big bang the universe was still largely devoid of chemical elements, which formed in the cosmos only bit by bit. Hydrogen and helium were present a few minutes after the big bang around 13.7 billion years ago. But it took several hundred million years before heavier elements appeared on the cosmic stage. First, hot stars capable of breeding elements up to iron by nuclear fusion had to form from the distributed matter via gravitation. The heavier elements, e.g. platinum, gold or lead are then created primarily in stellar explosions.
The cosmic mix of elements ultimately found its way into our solar system 4.5 billion years ago. That is why we can breathe oxygen and exhale carbon dioxide today, and why iron flows in our veins.
To better understand how the elements in the universe formed and to shed light on the myriad details of the processes, nuclear physicists and astronomers at the Excellence Cluster Universe collaborate closely. In their work, they also look at isotopes that decay after short periods of time. Isotopes are atomic nuclei of the same element that differ merely in their atomic weight. The difficulty to synthesize tin-100 is an example of such an isotope. Artificially producing this special nucleus and determining its properties was regarded as the “holy grail” of nuclear physics. Researchers from the Excellence Cluster Universe, in collaboration with the GSI Helmholtzzentrum für Schwerionenforschung in Darmstadt have now succeeded in doing just that. They now present the results of their work in the renowned scientific journal Nature (“Superallowed Gamow-Teller Decay of the Doubly Magic Nucleus Sn-100” by Hinke et al., Nature 486, 2012).
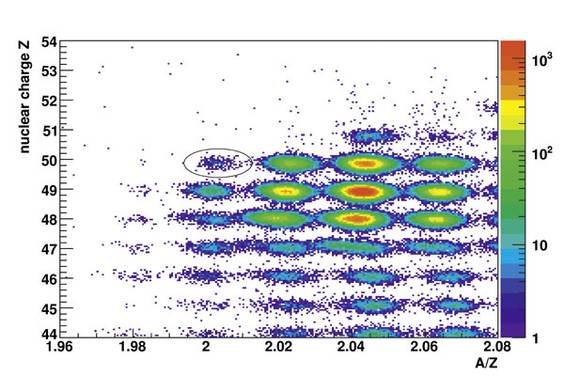
Tin-100 is different in that it has fewer neutrons, electrically neutral particles in atomic nuclei. Normal tin has at least 112 particles in its nucleus: 50 protons, positively charged particles, and 62 neutrons. The neutrons act as a kind of buffer between the electrically repelling protons, thereby preventing normal tin from decaying. Tin-100 has fewer neutrons: 50 to be exact. The buffer is thus weaker and tin-100 decays quickly as a result.
What makes tin-100 so special is the fact that it has exactly the same number of protons as neutrons, i.e. 50:50. And 50 is a magic number when it comes to atomic nuclei. Further neutrons are relatively weakly bound and additional protons remain unbound. Nuclear physicists refer to this property as “doubly magic.”
Since the atomic nucleus tin-100 is so unstable, it is very difficult to produce and verify. In an experiment, xenon-124 ions were shot onto a plate of beryllium at nearly the speed of light, thereby shattering into a variety of fragments that continue on their trajectories and can thus be identified. Scientists at the TU München developed novel particle detectors for the experiment, in addition to the standard technology at the GSI. Even though the first tin-100 nuclei were created and verified as early as the mid 1990s at the GSI and a facility in France, the real breakthrough did not come until now.
In the experiment at the GSI that lasted for three weeks the nuclear physicists have produced 259 tin-100 nuclei and investigated their decay. The protons in tin-100 are prone to beta decay, i.e. they transform into a neutron, a positron and a neutrino. This changes the number of protons in the nucleus (corresponding to the atomic number), resulting in an isotope of the element indium, which is located to the left of tin in the periodic table. The researchers were able to measure the half-life and the decay energy, thereby confirming something that had long since been postulated: tin-100 has the fastest beta decay transition of all atomic nuclei. The scientists also determined the energy of the gamma rays that are released when the excited residual nucleus settles down. This pioneering work was first presented in the scientific journal Nature on June 21, 2012. The lead author, Dr. Christoph Hinke, was honored with the PhD Award 2011 of the Excellence Cluster Universe for his work.
Understanding the properties of tin-100 is of great interest to particle and astrophysicists . During explosions at the surface of compact stars, increasingly heavier elements are formed by the successive capture of protons (in so-called rp processes). This is how nature creates the nucleus tin-100. Understanding the properties of tin-100 helps physicists understand what precisely is happening in the cosmos when heavy nuclei are synthesized in this way. In fact, the physicists may even be able to deduce the mass of neutrinos from a profound understanding of this decay.
A further improvement of the experiment is scheduled for the next spring at the RIKEN research center in Japan. The beam intensity at RIKEN is higher in the mean time, allowing even more precise measurements.